Ira M Longini, Yang Yang, Thomas R Fleming, César Muñoz-Fontela, Rui Wang, Susan S Ellenberg, George Qian, M Elizabeth Halloran, Martha Nason, Victor De Gruttola, Sabue Mulangu, Yunda Huang, Christl A Donnelly, Ana-Maria Henao Restrepo
Clinical Trials
July 22, 2022
ABSTRACT
Background:
The threat of a possible Marburg virus disease outbreak in Central and Western Africa is growing. While no Marburg virus vaccines are currently available for use, several candidates are in the pipeline. Building on knowledge and experiences in the designs of vaccine efficacy trials against other pathogens, including SARS-CoV-2, we develop designs of randomized Phase 3 vaccine efficacy trials for Marburg virus vaccines.
Methods:
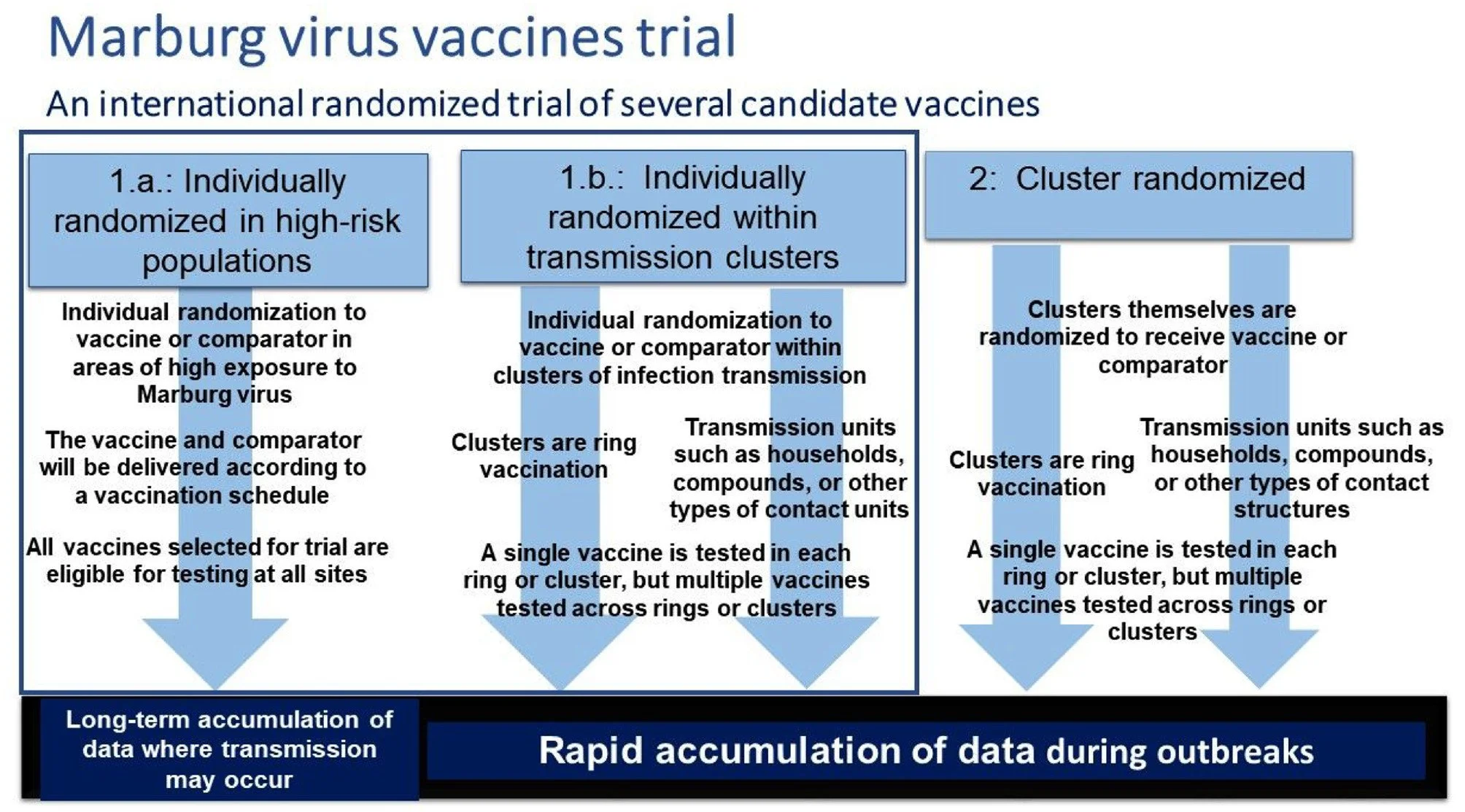
A core protocol approach will be used, allowing multiple vaccine candidates to be tested against controls. The primary objective of the trial will be to evaluate the effect of each vaccine on the rate of virologically confirmed Marburg virus disease, although Marburg infection assessed via seroconversion could be the primary objective in some cases. The overall trial design will be a mixture of individually and cluster-randomized designs, with individual randomization done whenever possible. Clusters will consist of either contacts and contacts of contacts of index cases, that is, ring vaccination, or other transmission units.
Results:
The primary efficacy endpoint will be analysed as a time-to-event outcome. A vaccine will be considered successful if its estimated efficacy is greater than 50% and has sufficient precision to rule out that true efficacy is less than 30%. This will require approximately 150 total endpoints, that is, cases of confirmed Marburg virus disease, per vaccine/comparator combination. Interim analyses will be conducted after 50 and after 100 events. Statistical analysis of the trial will be blended across the different types of designs. Under the assumption of a 6-month attack rate of 1% of the participants in the placebo arm for both the individually and cluster-randomized populations, the most likely sample size is about 20,000 participants per arm.
Conclusion:
This event-driven design takes into the account the potentially sporadic spread of Marburg virus. The proposed trial design may be applicable for other pathogens against which effective vaccines are not yet available.